Happy holidays and New Year!
FoodPolitics.com will be on vacation until January 2. All the best for a wonderful holiday season.

********
For 30% off, go to www.ucpress.edu/9780520384156. Use code 21W2240 at checkout.

FoodPolitics.com will be on vacation until January 2. All the best for a wonderful holiday season.

********
For 30% off, go to www.ucpress.edu/9780520384156. Use code 21W2240 at checkout.

Let’s start with this good thought:

The Farm Bureau has released its annual survey of the cost of Thanksgiving dinners, and the results will not surprise anyone who has been to a grocery store lately: up by a whopping 20%.


Food Politics will be back on Monday. Enjoy the holiday!
***********
For 30% off, go to www.ucpress.edu/9780520384156. Use code 21W2240 at checkout.

Here’s a report worth reading:

For reasons of history—and, alas, racism—laws requiring minimum wages do not apply to restaurant workers, farm workers, and home employment workers, all mostly people of color.
This report focuses on restaurant workers who depend on tips:
Unique among pay inequities, the subminimum wage for tipped workers was an original pay gap created intentionally to deny Black women any wage at all, forcing them to live on tips. This original and intentional pay inequity has been compounded over the last 160 years since Emancipation by ongoing inequities in hiring by employers and tipping and harassment by customers — resulting in an unlivable situation for Black women. The fact that Black women persist in the restaurant industry is a testament to many of these workers’ pride in their work as hospitality professionals who deserve to be remunerated as such.
The report offers three key findings:
It presents data arguing for having minimum wage laws apply to all workers. Seven states have passed such laws; the rest need to.
Trick or Treat? You can’t make this stuff up. CORRECTION: No you can’t. It’s a fake. Busted.

On the brighter side…
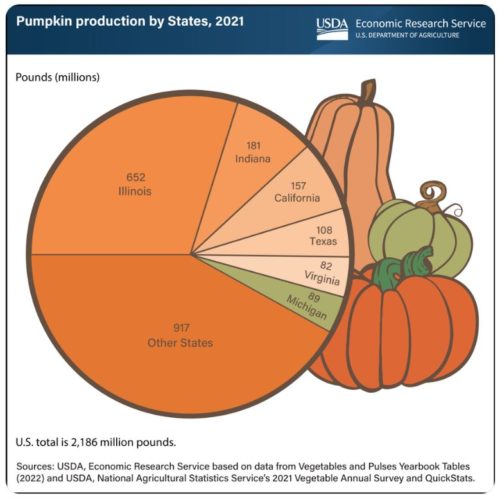
Happy Halloween!
***********
For 30% off, go to www.ucpress.edu/9780520384156. Use code 21W2240 at checkout.

I subscribe to the British-based newsletter, Food Navigator. It occasionally publishes roundups of articles on specific topics. Here’s a sample of articles about current happenings in the meat industry.
************
Coming soon! My memoir, October 4.
For 30% off, go to www.ucpress.edu/9780520384156. Use code 21W2240 at checkout.

Unless you are deeply involved in its planning, which I am not, it’s hard to know what’s going on with the planned-for-September (date not yet set) White House Conference on Hunger, Nutrition, and Health.
The official information about the conference is on the Health.gov website. Its goal:
End hunger and increase healthy eating and physical activity by 2030, so that fewer Americans experience diet-related diseases like diabetes, obesity, and hypertension.
Its pillars:
Here’s how you can weigh in: host your own session (explained in this press release.
Here are the questions for consideration:
Tufts University is leading an effort to generate actionable recommendations to inform the conference. Its work is described here.
This is all I know at the moment. I went to the listening session at Gracie Mansion a couple of weeks ago but have not heard what, if anything, came out of it.
Food prices are rising. The American Farm Bureau keeps score.
Every year, the American Farm Bureau, with the help of volunteer shoppers around the country, calculates the average cost for a July 4th cookout. This year, it will cost about $70 to feed ten people. That’s a 17% increase compared to a year ago. Inflation, ongoing supply chain disruptions, and the war in Ukraine are all contributing to the substantial increase in food prices.

And stay safe! (Food Safety News has the details on how to avoid food poisonings).
The watchdog Environmental Working Group has analyzed the approval process for new food chemicals. Its disturbing conclusions:
Nearly 99 percent of all food chemicals introduced since 2000 were greenlighted for use by the food and chemical industry,…not by the Food and Drug Administration, the agency responsible for ensuring food is safe.
That’s because food and chemical companies exploited a loophole in the law allowing them to decide which chemicals are safe to consume, contrary to what Congress intended when it enacted food chemical laws in 1958….for 756 of 766 new food chemicals added to the food supply since [2000], or 98.7 percent, these companies have exploited a loophole for substances that are “generally recognized as safe,” or GRAS. The loophole lets them – not the FDA – decide a substance is safe.
The data:
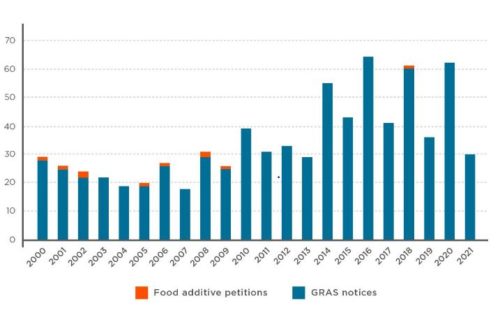
Do we care? I think we should.
EWG deserves thanks for keeping an eye on this issue.