Happy FoodPolitics Valentine’s Day!
And don’t miss Food Corps‘ gift of Veggie Valentine cards. Here’s an example:

*******
For 30% off, go to www.ucpress.edu/9780520384156. Use code 21W2240 at checkout.



And don’t miss Food Corps‘ gift of Veggie Valentine cards. Here’s an example:

*******
For 30% off, go to www.ucpress.edu/9780520384156. Use code 21W2240 at checkout.

Astaxanthin is the dye used by growers of farmed salmon to give the fish a pink color. They do this by matching the desired amount of the dye to this SalmoFan template.
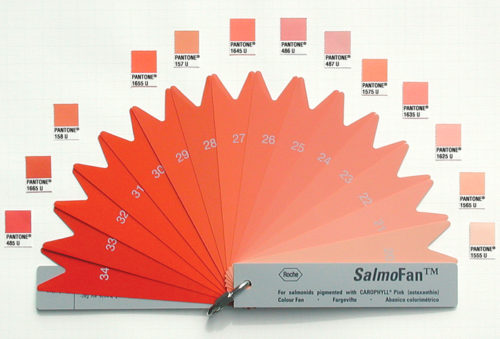
Astaxanthin, claim its makers, is an antioxidant and, therefore, good for you.
First the public relations: Screen shot: Astaxanthin reduces work-induced visual stress in people aged over 40 – BGG trial: Astaxanthin supplementation has been found to reduce visual display terminal (VDT) work-induced visual stress in people aged over 40, according to a new Japan trial sponsored by supplier BGG Japan. Read more
As for the actual research:
The study: Effects of diet containing astaxanthin on visual function in healthy individuals: a randomized, double-blind, placebo-controlled, parallel study. Takahiro Sekikawa, Yuki Kizawa, Yanmei Li, Naoki Miura. J Clin Biochem Nutr. https://doi.org/10.3164/jcbn.22-65.
Results: In participants aged ≥40 years, corrected visual acuity of the dominant eye after visual display terminal work at 6 weeks after intake demonstrated a higher protective effect of astaxanthin in the astaxanthin group vs the control group (p<0.05). In participants aged <40 years, no significant difference was seen between the astaxanthin and control groups. Moreover, no significant difference was found in functional visual acuity and pupil constriction rate between the astaxanthin and control groups [my emphasis].
Conclusion: These results suggest astaxanthin reduces oxidative stress caused by visual display terminal work. Age-related reduction in ciliary muscle strength is likely the main detractor of visual acuity. Correspondingly, astaxanthin reduced visual display terminal work-induced visual stress in the middleaged and elderly.
Conflict of interest: This trial was sponsored by BGG Japan Co., Ltd., which provided all costs for the implementation of the trial. TS and YK are employees of BGG Japan Co., Ltd. YL is an employee of Beijing Gingko-Group Biological Technology Co., Ltd., which belongs to the same company group as BGG Japan Co., Ltd. NM received funding from BGG Japan Co., Ltd. to provide medical services to the trial participants.
Comment: BGG makes astaxanthin products. This is a company-sponsored study conducted by researchers employed or paid by the company. It is an excellent example of interpretation bias—putting a positive spin on results that are marginally significant in some groups but not others, or null.
*******
For 30% off, go to www.ucpress.edu/9780520384156. Use code 21W2240 at checkout.

Oh no! US Snack Index reveals consumers’ fear of running out of snacks during the Super Bowl
This has to be a commentary on where we are as a food nation.
This survey of Super Bowl fans found that a whopping 49% “would rather see their team lose than have to sit through the game without snacks.”
OK. The survey was conducted by Frito-Lay, a not exactly disinterested observer, but still.
It makes clear that the Super Bowl is only peripherally about sports. It is about eating junk food.
You don’t believe me? Try:
As for Super Bowl food politics, try getting your head around the danger Mexican truckers face bringing avocados North for your guacamole.
Enjoy the game!
*******
For 30% off, go to www.ucpress.edu/9780520384156. Use code 21W2240 at checkout.

Thanks to Lisa Young for this:

The average price of a dozen eggs goes up and up. It now averages $4.25 a dozen, and that’s for the cheapest kinds.
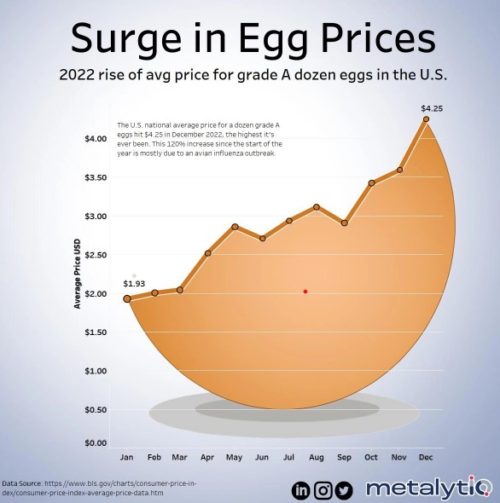
It could get worse. Avian flu infects animals as well as birds and could infect us.
How’s that for a cheery thought.
Small egg farms, anyone?
Or chickens as art, per National Geographic?
********
For 30% off, go to www.ucpress.edu/9780520384156. Use code 21W2240 at checkout.

So many readers have asked me to comment on the American Academy of Pediatrics’ Clinical Practice Guideline for the Evaluation and Treatment of Children and Adolescents With Obesity that I thought I ought to say something about it.
The guideline report is so long and detailed that I cannot imagine anyone actually reading it. I started with the introduction, which summarizes basic facts.
What got press attention—and the attention of readers of this blog—is the AAP’s endorsement of drug and bariatric surgical treatment of obese children.
I cut right to the chase and looked at Appendix I, which gives the AAP’s algorithm for deciding on treatment options.
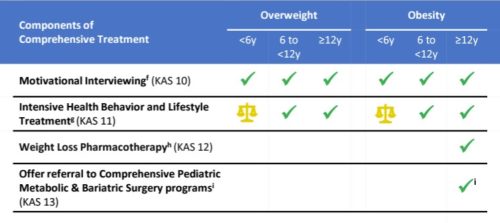
The report’s major conclusions expand on this chart (the report does not define KAS, but I think it means Knowledge, Attitudes, and Skills):
KAS 9. Pediatricians and other PHCPs should treat overweight (BMI ≥ 85th percentile to <95th percentile) and obesity (BMI ≥ 95th percentile) in children and adolescents, following the principles of the medical home and the chronic care model, using a family-centered and nonstigmatizing approach that acknowledges obesity’s biologic, social, and structural drivers.
KAS 10. Pediatricians and other PHCPs should use motivational interviewing (MI) to engage patients and families in treating overweight (BMI ≥ 85th percentile to <95th percentile) and obesity (BMI ≥ 95th percentile).
KAS 11. Pediatricians and other PHCPs should provide or refer children 6 y and older (Grade B) and may provide or refer children 2 through 5 y of age (Grade C) with overweight (BMI ≥ 85th percentile to <95th percentile) and obesity (BMI ≥ 95th percentile) to intensive health behavior and lifestyle treatment. Health behavior and lifestyle treatment is more effective with greater contact hours; the most effective treatment includes 26 or more hours of face-to-face, family-based, multicomponent treatment over a 3- to 12-mo period.
KAS 12. Pediatricians and other PHCPs should offer adolescents 12 y and older with obesity (BMI ≥ 95th percentile) weight loss pharmacotherapy, according to medication indications, risks, and benefits, as an adjunct to health behavior and lifestyle treatment.
KAS 13. Pediatricians and other PHCPs should offer referral for adolescents 13 y and older with severe obesity (BMI ≥ 120% of the 95th percentile for age and sex) for evaluation for metabolic and bariatric surgery to local or regional comprehensive multidisciplinary pediatric metabolic and bariatric surgery centers.
What is not in this guideline is anything that addresses the social determinants of childhood obesity. What we have here is a focus on treating the symptoms, but getting nowhere near the cause.
It is difficult for someone like me who is not affected by those determinants to even imagine how drugs and surgery could be thought even remotely acceptable for children, even those over the age of 12, but I am not treating these kids.
Providers who do treat obese children tell me they are relieved to be able to offer options that might help kids achieve healthier weights.
As I see it, these should be absolute last resorts if used at all. And this is without even getting into issues of cost or our dysfunctional health care system.
In public health terms, drugs and surgery are “downstream” solutions to a problem that began way upstream with all those societal determinants.
If ever we needed upstream approaches, chldhood obesity is a prime example.
Upstream means policy changes that make healthy eating more appealing, accessible, and affordable That’s what pediatricians need to be calling for.
This AAP report deliberately separates treatment from prevention. It promises a discussion of prevention in a subsequent report. I hope it is as hard hitting as any AAP report has ever been.
If childhood obesity teaches us anything, it is that our society needs to change in ways that are healthier for our children.
Additional supporting documents
Thanks to all the people who wrote me about this. Much appreciated.
*******
For 30% off, go to www.ucpress.edu/9780520384156. Use code 21W2240 at checkout.

Last week, the FDA announced its proposed Redesign of Human Foods Program to Enhance Coordinated Prevention and Response Activities. You can also watch this announcement on video.
This action comes in the wake of:
The FDA proposal comes with a vision of how the redesign would work.
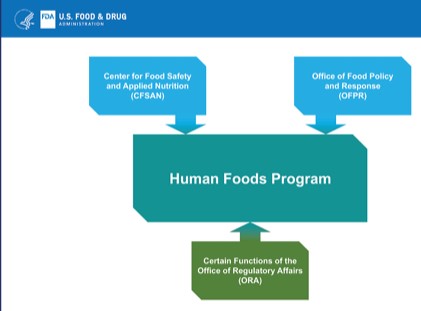
The complaints and pressures argued for appointing a senior FDA official in charge of all FDA food programs and units. Instead, the FDA proposes to create a Human Foods Program encompassing some, but not all, FDA food units.
Most prominently missing is the Center for Veterinary Medicine which deals with food for farm animals and pets. These, however, fully participate in the food system for humans; they eat most of US corn production and loads of byproducts of human food production. The systems for humans and animals are inextricably linked.
The organizational chart will look like this. It splits the food units and connects them with dotted lines. Good luck with that. That was precisely the problem with the previous organization.
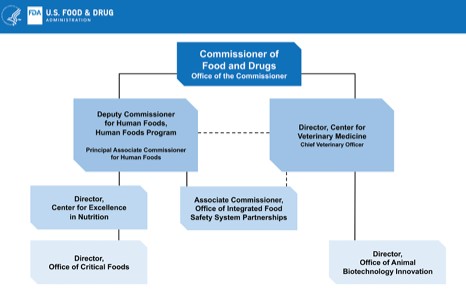
This looks better than the previous organization chart but falls far short of the authority needed to solve the nation’s food safety problem.
What’s needed?
Hey, I can dream.
In the meantime, let’s see how the FDA’s new structure goes and who it hires into that key position in charge of human foods.
********
For 30% off, go to www.ucpress.edu/9780520384156. Use code 21W2240 at checkout.

I saw this on NutraIngredients.com, one of those newsletters I read regularly: Study: Mulberry extract shrinks blood sugar spike by 40%.
That headline was all I needed: Who paid for this?
Fortunately, the study was readily available: Mulberry leaf extract improves glycaemic response and insulaemic response to sucrose in healthy subjects: results of a randomized, double blind, placebo‑controlled study. Nutr Metab (Lond). .2021 Apr 15;18(1):41.
Conclusion: “Mulberry leaf extract can be used as part of lifestyle changes that may lead to healthy blood glucose levels.”
Acknowledgements: The authors would like to thank Dr Chen Xie (CX) and Mrs Hongwen Yu (HY) for technical input during the conceptualization of the study and editorial commentary on the draft publication. CX and HY are both employees of Phynova Group Limited.
Funding: The work in this study was funded by Phynova Group Ltd, a privately held company that is the developer of Reducose® mulberry leaf extract. Phynova was involved in the study design and manuscript preparation but had no role in data collection and analysis.
Competing of interest: AG is an employee of Phynova Group Limited, the funder of the study. He was involved in the development of the product, design of the study and drafting of the manuscript, but was not involved in the collection, analysis or interpretation of data. PST, HL, and LA have declared they have no conflicts of interest.
Comment: This degree of industry involvement turns this study into industry-funded marketing research, no matter how beautifully the science is conducted. Please refer to my book, Unsavory Truth, for references to the studies backing up that statement.
********
For 30% off, go to www.ucpress.edu/9780520384156. Use code 21W2240 at checkout.

The Federal Trade Commission has issued a Health Products Compliance Guide.

This amazing publication takes on the Dietary Supplement Health and Education Act of 1994. That act effectively removed the FDA from regulating dietary supplements. It also allowed supplement labels to make “structure/function” claims that the supplement supported some structure or function of the body, whether or not there was much in the way of scientific evidence to back up those claims (in contrast, FDA-authorized claims must be scientifically substantiated).
Now the FTC is saying:
Marketers of health-related products, including dietary supplements, should be familiar with the requirements under both FDA law and FTC law that labeling and advertising claims be truthful, not misleading, and substantiated. The FTC approach generally requires that health-related claims be backed by competent and reliable scientific evidence substantiating that the representations are true.
Marketers cannot suggest unsubstantiated benefits, safety, or other characteristics.
Example 4: An ad for an infant formula states that an ingredient added to the formula can reduce the symptoms of colic. The ad includes an unrelated chart from a pediatric journal showing that, as a general principle, the length of time that colicky babies cry tends to decrease over the first 12 weeks of life…Using the graph in an ad for the infant formula likely implies that the formula, rather than the babies’ ages, causes the decrease in crying time.
Claims have to be qualified.
Example 10: An energy drink contains an ingredient that, when consumed daily over an extended period, can result in a significant increase in blood pressure. Even absent any representation about the product’s safety, the marketer should disclose this potentially serious risk.
Qualifying information must be clear and straightforward.
Example 13: A company has results from two studies suggesting that its supplement helps to maintain healthy cholesterol levels. There are, however, significant limitations to each of the studies… The company makes a claim in advertising that “promising, preliminary scientific studies show that our product may be effective in reducing cholesterol.” The use of the words “promising,” “preliminary,” and “may” is unlikely to sufficiently convey the limitations of the science.
Assertions about the strength of evidence must be accurate.
Example 16: An ad for a supplement includes the statement “Scientists Now Agree!” in discussing the product’s benefit. This statement likely conveys to consumers that the state of science supporting the benefit has reached the level of scientific consensus. Unless the advertiser possesses evidence demonstrating that scientists have reached that consensus, the claim is false.
Marketers must consider the totality of the science.
Example 30: An advertiser wants to claim that a supplement will substantially reduce body fat. The advertiser has two controlled, double-blind studies showing a modest but statistically significant loss of fat at the end of a six-week period. However, there is an equally well-controlled, double-blind 12-week study showing no statistically significant difference between treatment and control groups. Assuming other aspects of methodology are similar, the studies taken together suggest that, if the product has any effect on body fat, it would be very small and may not persist over time. Given the totality of the evidence, the claim is unsubstantiated.
Here’s the press release.
The bottom line: The FTC is requiring evidence for health claims on supplements.
This will affect claims for thousands of supplement products.
Enforcement anyone? This should be fun to watch.
********
For 30% off, go to www.ucpress.edu/9780520384156. Use code 21W2240 at checkout.
