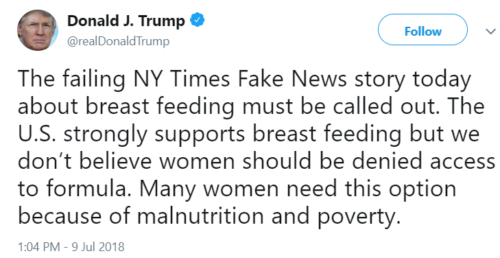
What’s up with infant formula?
DairyReporter.com has a Special Edition on infant feeding
Infant formula companies have a problem: the products are virtually identical in nutrient composition (they all have to meet the same FDA standards), babies only need them for the first year, and the number of babies is finite. From the formula industry’s perspective, the challenge is how to increase sales. Here’s how this industry is managing this challenge.
Where next for infant nutrition?
In this special edition, we take a look at the infant formula sector, which far from being static is changing through novel ingredients, smart packaging, the explosion in plant-based products, and production of breast milk from cells.
- Making baby formula transparent with Danone’s Track & Connect: Packaging with QR codes and a new digital platform make up Danone’s Track & Connect service for baby formula products. It is available in China, with launches in France, Germany, Australia and New Zealand soon… Read
- How to trigger a growth spurt in infant nutrition: Consumers could be forgiven for believing that innovation in infant nutrition is less radical or slower than it is in other parts of the nutrition industry… Read
- Alternatives to traditional infant nutrition hit US market: Market trends and unique consumer needs in infant nutrition have producers thinking outside the realms of tradition. New launches from Else Nutrition and Biomilq have recently hit the US, forming new categories… Read
- TurtleTree Labs secures funding for production of milk from cells: TurtleTree Labs, the Singapore-based company working with biotechnology to create the full nutritional content of milk, has announced it has completed its pre-seed funding round… Read
- Stricter EU infant formula marketing rules to come into effect: New EU food law designed to further restrict the advertising and marketing of infant formula are to come into force on 22 February in a victory for campaigners critical of current guidelines… Read
- Combating cow’s milk protein allergy: Nestlé Health Science China introduces two new FSMPs brands: Two of Nestlé Health Science China’s infant nutrition products for youngsters with cow’s milk allergies have obtained Food for Special Medical Purposes (FSMPs) status in the country… Read
- Catering for different life stages: What’s next for China’s A2 milk industry in 2020? China’s A2 milk sector is likely to see the rise of more products catered to different life stages and specific consumers groups, beyond just infant nutrition, as more domestic companies join in the competition… Read