Letter to FDA on front-of-package labels
I signed a letter organized by the Center for Science in the Public Interest calling on the FDA to do more to research front-of-package labels.
This is in response to the FDA’s announcement of what it plans to test in developing a front-of-package labeling scheme.
We asked the FDA for specific additions to the research proposals, among them this one:
- Consider testing additional High In scheme designs with attention-grabbing features like these:
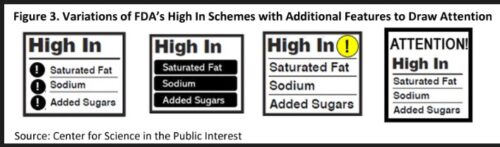
We noted that the FDA states three goals for the research:
- Participants’ ability to correctly interpret the nutritional profile of the product
- The speed at which participants make their decisions
- Whether or not participants search for more information to answer the question (i.e., whether they click a link to view the Nutrition Facts label)
We argued that
Of the three outcomes, we believe that participants’ ability to correctly interpret the nutritional profile of the product is the most important [because it is the only one that is independently and objectively desirable. In contrast, the desirability of faster decision-making is dependent on whether the decision is correct, and it is unclear what would be the more desirable outcome with respect to searching for the Nutrition Facts label. Searching for the Nutrition Facts label could be positive (if the labeling scheme spurs consumers to learn more about the product’s nutrition information and ingredients) or negative (if the labeling scheme is not noticeable or confusing and thus participants need to seek more information).
Front-of-package labeling has been in the works for a long time. It’s great the FDA is getting to it.

