I don’t track legislation very carefully because bills change so much between the time they are proposed and actually pass. But I keep getting asked about the bills that seem to have the best chance this year, H.R. 2749 (which has just been passed by the House) and its equivalent in the Senate, S. 510 (still in the works). The bills are quite similar. Both aim to fix the FDA. Neither aims to fix the system, so forget about combining the food safety functions of USDA and FDA into one agency. The bills bring the FDA’s rules closer to those of USDA, as they propose science-based food safety standards (much like HACCP) from farm to table. Best, they give the FDA recall authority as well as a few other goodies.
The bills themselves are miserable to read and it is hard to believe that anyone in government does. That is why the Congressional Research Service (CRS) does summaries that even legislators can understand. CRS researchers have now produced blessedly short and hopefully accurate summaries of the House bill as well as the Senate bill.
As my contribution to the cause of clarity, I have done a quick edit of the CRS summaries, with comments in Italics. The links above are to the original bills so you can plow your way through them to see if this does them justice. Enjoy!
THE HOUSE BILL, H.R. 2749, requires each food facility to:
(1) Conduct a hazard analysis, (2) Implement preventive controls, and (3) Implement a food safety plan. [This sounds like HACCP, although they aren’t calling it that. I vote yes]
Requires FDA to:
(1) Issue science-based performance standards to minimize the hazards from foodborne contaminants [this means HACCP or its equivalent, and about time too],
(2) Establish science-based standards for raw agricultural commodities [this means some version of farm-t0-table HACCP, long awaited],
(3) Inspect facilities at a frequency determined pursuant to a risk-based schedule [this is an admission that the FDA can’t handle the work load; it will focus on products most likely to be contaminated]
(4) Establish a food tracing system [this will help identify where foods come from]
(5) Assess fees relating to food facility reinspection and food recall [make companies pay for all this, I hope in a way that avoids conflicts of interest],
(6) Establish a program for accreditation of laboratories that perform analytical testing of food for import or export [can’t believe we don’t already have this, but that’s why we need this legislation].
Authorizes FDA to:
(1) Order an immediate cessation of distribution, or a recall, of food [recall authority at last!]
(2) Establish an importer verification program [accountability for importers, at last!]
(3) Quarantine food in any geographic area within the United States [they can’t do this now?].
Defines the term “color additive” to include carbon monoxide that may affect the color of fresh meat, poultry products, or seafood [this will have to meet food additive regulations].
Requires country of origin labeling on food, and annual registration of importers [Yes!].
Provides for unique identifiers for food facilities and food importers [so FDA actually knows who they are].
Deems a food to be adulterated if an inspection is delayed or refused [Yes!].
Requires FDA to establish a corps of inspectors dedicated to inspections of foreign food facilities [Amazing that we don’t already have this].
Reorganizes the FDA field laboratories and district offices [Could this possibly be a euphemism for closing some?].
Gives the FDA Commissioner subpoena authority [Yes!].
Establishes whistleblower protections [OK].
THE SENATE BILL, S. 510, is pretty much the same except that it addresses food bioterrorism [fortunately, a rare event so far]. In addition to most of what is in the House bill, it requires HHS and USDA to prepare the National Agriculture and Food Defense Strategy [If this is done right, it ought to promote the safety of domestic foods and imports].
It also requires FDA to:
(1) Identify preventive programs and practices to promote the safety and security of food [worries about food bioterrorism again];
(2) Promulgate regulations on sanitary food transportation practices [good idea];
(3) Develop a policy to manage the risk of food allergy and anaphylaxis in schools and early childhood education programs [I’m not sure how this got in here]
Requires FDA and CDC to enhance foodborne illness surveillance systems [Good idea].
Requires EPA to assist state, local, and tribal governments in preparing for, assessing, decontaminating, and recovering from an agriculture or food emergency [in the military sense of food security].
There is much, much more in these bills. Bill Marler, who has actually read the bills, has produced his own summary, which includes definitions and more. If you are wondering what implementation of these bills might cost, the Congressional Budget Office has done an analysis: a mere $2 billion.
It’s hard to know how seriously to take all this until we see what Congress actually does when it gets back to work. Stay tuned.


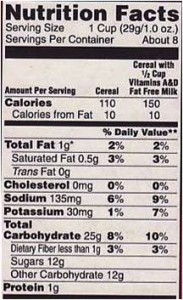 OK. I understand that companies want to market their processed foods, but I cannot understand why nutrition societies thought it would be a good idea to get involved with this marketing scheme. It isn’t. The American Society of Nutrition gets paid to manage this program. It should not be doing this.
OK. I understand that companies want to market their processed foods, but I cannot understand why nutrition societies thought it would be a good idea to get involved with this marketing scheme. It isn’t. The American Society of Nutrition gets paid to manage this program. It should not be doing this.


