In the past few weeks, I’ve been sent several questions asking my opinion of food professionals’ relationships with food companies. I thought I would deal with them at one time (all are edited for succinctness and clarity):
Q. I am a food science student looking into career options in the food industry. I love food science and truly believe that processing food is a good idea that can positively impact the planet and its people. I want to do something worthwhile,, but I still need to eat. Can’t the food industry be changed from the inside? Can’t you advise good companies, people, or places where I could start my search?
A. I have met social entrepreneurs who strongly believe that businesses can be ethical, do good, and still make heaps of money. Maybe so. If you are going to try that route, I think it essential that the company be family or cooperatively owned, and not publicly traded. You might take a look at food companies incorporated as Benefit Corporations. These are now authorized in about half the states to consider the interests of all stakeholders, not just shareholders, when making decisions. They are different from B corporations certifying companies that meet certain sustainability criteria.
Many companies work hard to reduce their environmental impact. But the real question is what they are doing about health impact. Are they going overboard on health claims? Are they marketing to children? These are questions I’d want to ask. In your shoes, I’d start by looking at companies making products that you like, feel good about, and would be proud to be associated with. And then take a closer look at how the companies operate. Working for food companies is always a good learning experience, but if you really want to change the world, you might be better off with a nonprofit agency.
Q. I’m a graduate student in nutrition and I would like to know what recommendations you may have for students to navigate conflicts of interest with food companies when beginning a career. I intend to pursue an academic career but am concerned that my credibility as a scientist could be compromised by my participation in industry-funded publications and research.
A. It’s great that you are asking such questions. From the standpoint of ethics, that’s an important first step. You should most definitely publish your research, no matter how it is funded. Be sure to disclose potential sources of funding bias and conflicts of interest. While you are doing your research, you can take special care to control for potential biases—conscious and unconscious—in your study design, conduct, and interpretation to ensure that they are not influenced by the funder. In searching for jobs, you might consider those in academia, government, and NGOs that are less likely to require you to compromise principles. Finding such jobs may not be easy, but you will be OK if you are always ethically transparent and as straightforward about biases as it is possible to be.
Q. Do you believe that relationships between the food industry and nutrition professional organizations like the Academy of Nutrition and Dietetics (AND) and the Association for Nutritional Science (ASN) are problematic? Why?
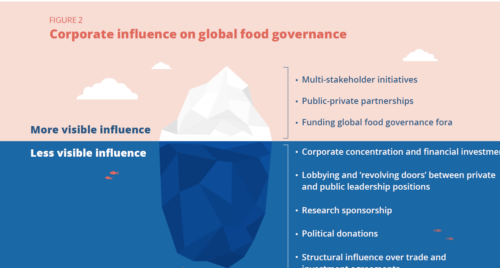
A. Sponsorship by food product makers puts nutrition professionals in conflict of interest. Nutritionists ought to be advising clients and the public about what to eat to stay healthy and prevent chronic disease. This necessarily means promoting consumption of some foods but discouraging consumption of others. Nutritionists cannot speak truth to clients and protect corporate sponsorship at the same time. If nothing else, food industry sponsorship gives the appearance of conflict of interest and makes AND and ASN appear as arms of food company marketers. But it also affects—or appears to affect–AND ’s and ASN’s positions on key issues in nutrition and health. Overall, financial relationships between these organizations and their food industry sponsors undermines the credibility of their positions on food issues.
Q. What sort of changes do you think the Academy needs to make in order to make food industry relationships more beneficial to its members and the public overall?
A. Nutrition and food professional organizations need to establish a firewall between corporate sponsorship and content or opinion. This requires setting up rigorous guidelines for what food companies can and cannot expect from their donations. They should not, for example, be permitted to sponsor content sessions at meetings, not least because opinions expressed at sponsored sessions rarely appear objective. The organizations should have complete control over how and where corporate donations and company logos are used.
Q. How can relationships between health professionals and the food industry be beneficial for public health overall?
A. The role of health professionals is to give the best advice possible about diet and health. The role of food companies is to provide profits to shareholders. These goals are not the same and are only rarely compatible. In my experience, people who want to work for food companies to change corporate culture from within do so from good motives, but soon discover that corporate imperatives take precedence over health goals. If health professional organizations want their advice to be taken seriously, they must establish and adhere to rules and guidelines designed expressly to protect their integrity.
Q. I read your post on the revolving door, It seems to me that your underlying premise is the notion that any company that makes food is indicted as part of the big evil food conspiracy. Surely, you can’t really believe that.
A. Of course I don’t. But food companies are not social service agencies. Their job—their legal responsibility—is to continuously expand sales and distribute ever-increasing profits to shareholders. If they can do this and promote health at the same time, more power to them. But people would be healthier eating food, not food products. In our present system, products are far more profitable and the focus on them is rarely works in the interest of public health.
Food and nutrition professionals need to make a living. Unfortunately, jobs in industry pay better–and sometimes a lot better–than jobs in government or NGOs. That’s the real dilemma that underlies all of these questions.