Weekend thinking: The FDA v. salt
The FDA is once again asking food companies to voluntarily reduce the sodium in their products.
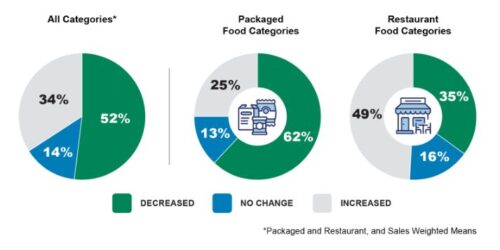
It says that 40% of food categories have done just that.
Prior to 2021, consumer intake was approximately 3,400 milligrams per day on average, far higher than the limit recommended by the Dietary Guidelines for Americans of 2,300 milligrams per day for those 14 years and older.
If finalized, the new set of voluntary targets would support reducing average individual sodium intake to about 2,750 milligrams per day. This reduction is approximately 20% lower than consumer intake levels prior to 2021.
it has published a report on this progress.
A quick reminder: salt is 40% sodium. The Dietary Guidelines upper limit target of 2300 mg/day sodium means nearly 6 grams of salt per day, or 1.5 teaspoons.
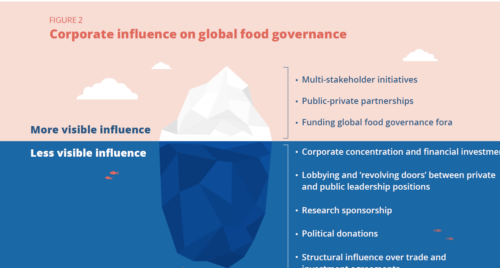
As for why this matters, Sodium Reduction Is A Proven Strategy That Saves Lives—More Work Is Needed To Hold Industry Accountable.
In 2016, the Food and Drug Administration (FDA) embarked on a sodium reduction strategy, only to meet repeated political hurdles…there has been little industry engagement, minimal public reporting, and no consequences if targets are not achieved.
Salt reduction across the entire food supply is the only measure that will help people reduce sodium intake. This issue has been around for a long time.
Voluntary reduction is nice, but does not go nearly far enough and it can always be reversed.
The FDA could and should do more.
OK, granted. Political opposition to salt reduction is fierce—if foods aren’t salty enough, people might not buy them.
But the FDA also has a long history of protection of commercial interests, which it claims it cannot share because it is obliged to protect trade secrets. It’s time for that to change too.