Beech-Nut recalls, will stop selling, rice cereal
In the latest episode in the ongoing saga of high levels of arsenic in rice cereal marketed to babies, Beech-Nut says it is going out of the rice cereal business.
In addition to issuing the voluntary recall, Beech-Nut has also decided to exit the market for Beech-Nut branded Single Grain Rice Cereal. Beech-Nut is concerned about the ability to consistently obtain rice flour well-below the FDA guidance level and Beech-Nut specifications for naturally occurring inorganic arsenic.
Background: I have been posting about arsenic in rice since 2013. In 2015, I wrote about how
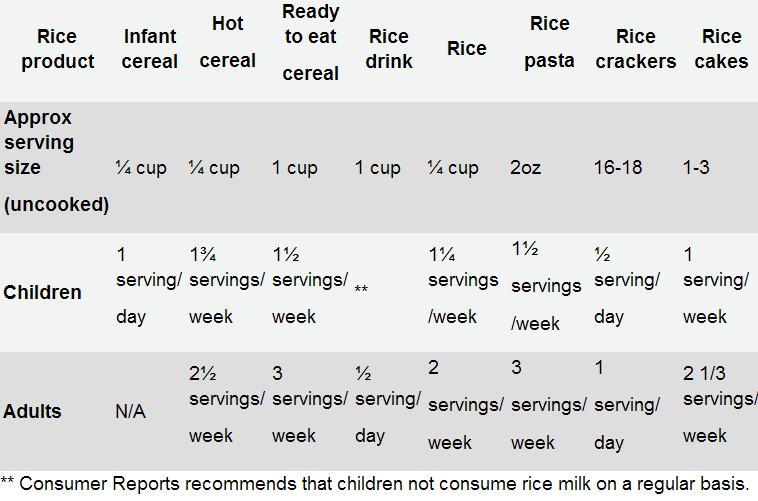
- Rice cereals contain higher-than-desirable levels of arsenic.
- Arsenic gets into rice from natural sources but also from arsenic pesticides.
- The FDA says these levels do not pose health problems.
- Consumer Reports recommends not feeding rice cereals to children.
- The U.S. Rice Federation said the CR recommendation was not supported by science.
In August 2020, the FDA finally set guidelines for upper limits of arsenic in baby foods. I wrote most recently—in May this year—about pressures on the FDA to set and enforce more rigorous standards and to go beyond its “Closer to Zero” plan to reduce heavy metals in baby foods.
Now the FDA announces the recall of Beech-Nut rice cereal with levels of arsenic that exceed those standards.
The Environmental Working Group asks if you should worry about arsenic in rice. Its answer: Yes.
Rice, it points out, is a “specifically risky crop. Eating less rice and foods with rice-based ingredients will decrease the amount of arsenic in your body.”
EWG also recommends government actions:
- Crop monitoring programs
- Soil testing
- Elimination of use of sewage sludge as fertilizer (this practice is forbidden in organic foods)
- Clear communication of risks
At long last, pressures to fix this problem are having an effect.
Lawsuits also might help, as indicated by this item.
Heavy metals in baby food: 86 lawsuits and counting as Beech-Nut decides to exit infant rice cereal category owing to inorganic arsenic concerns: At least 86 lawsuits have now been filed against firms named in the recent Congressional Subcommittee report on heavy metals in baby food including Beech-Nut Nutrition, which has just announced plans to exit the infant rice cereal category citing difficulties in sourcing rice flour that is consistently below FDA guidance levels for inorganic arsenic…. Read more