Are frozen foods the cause of the Covid-19 pandemic?
I can’t believe we are even talking about this, but the FDA, USDA, and CDC have just issued a rare joint statement addressing it [my emphases throughout].
After more than a year since the coronavirus disease 2019 (COVID-19) outbreak was declared a global health emergency, the U.S. Department of Agriculture, the U.S. Food and Drug Administration and the U.S. Centers for Disease Control and Prevention continue to underscore that there is no credible evidence of food or food packaging associated with or as a likely source of viral transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus causing COVID-19.
This, no doubt, is in response to statements from the World Health Organization team that visited Wuhan to determine the source of the virus causing Covid-19. This team has not yet issued its report, but members of the team have talked to reporters.
The researchers largely discounted the controversial theory that the virus accidentally leaked from a laboratory, and suggested that SARS-CoV-2 probably first passed to people from an animal — already a leading hypothesis among researchers. But the team also offered two hypotheses promoted by the Chinese government and media: that the virus, or its most recent ancestor, might have come from an animal outside China, and that once it was circulating in people, it could have spread on frozen wildlife and other cold packaged goods….Dominic Dwyer, a medical virologist at New South Wales Health Pathology in Sydney, Australia, and a member of the WHO team, says there is some evidence that the coronavirus could have spread on contaminated fish and meat at Chinese markets, and more details will be included in the written report.
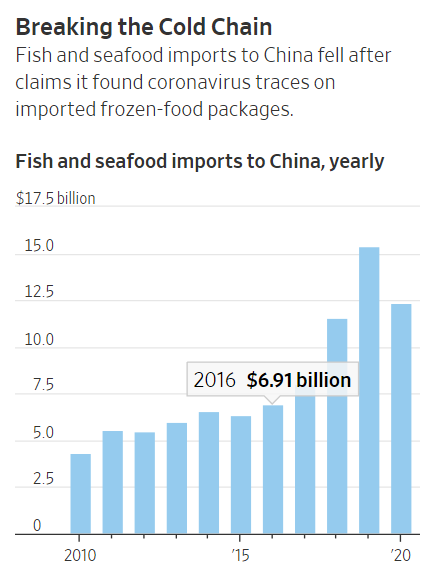
According to the Wall Street Journal,
Beijing has blamed frozen-food imports as one cause of a string of recent outbreaks, and it has introduced mandatory testing and disinfection of foreign goods, saying it found traces of the virus on packaging of products including American pork, Saudi shrimp and Brazilian beef.
This idea has had a profound effect on sales of frozen foods.
Everyone seems to agree that there are four key hypotheses to explain the origin of this particular Coronavirus:
- Direct animal vector
- Intermediary animal vector
- Laboratory accident
- Frozen food products,
The team could not identify a specific animal vector, dismissed the idea of a laboratory accident, but left open the possibility of frozen food.
The frozen food idea was suggested by the Chinese.
Several top Chinese scientists have further suggested that the Sars-CoV-2 virus that causes the Covid-19 disease may have arrived in the Huanan Seafood Wholesale Market in Wuhan city, the location of the world‘s first known outbreak, via frozen food imports, or what’s referred to as cold-chain transmission.
The WHO team’s report is considered a public relations win for the Chinese.
The W.H.O. team opened the door to a theory embraced by Chinese officials, saying it was possible the virus might have spread to humans through shipments of frozen food, an idea that has gained little traction with scientists outside China.… The virus was circulating in Wuhan several weeks before it appeared at the Huanan Seafood Wholesale Market, where some of the earliest clusters were initially reported, the experts said. It most likely emerged in bats and spread to humans through another small mammal, though the experts said they have not been able to identify the species.
The team, as I mentioned, considered a laboratory origin unlikely.
The team called for further investigation into the possibility of “cold chain” transmission, referring to the transport and trade of frozen food.
US Federal agencies don’t believe this for a minute; hence, their joint statement.
So if frozen foods are not at fault, and no animal vector can be conclusively identified, that leaves us with the dismissed-out-of-hand laboratory origin.
So what’s up with that? Wuhan, where the pandemic started, happens to be the site of a laboratory that works on Coronaviruses.
- New York Magazine has a long investigative report on this topic.
- Independent Science News has published several articles examining this possibility (the latest is here).
- US Right to Know has a reading list on Covid-19 origins
Why does the origin of the pandemic matter?
- If we don’t know how this one happened, how can we take steps to prevent the next one?
- And it matters a lot to the makers of frozen foods.

 What is this?
What is this?



