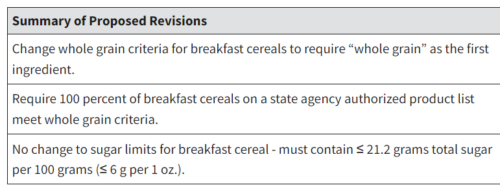
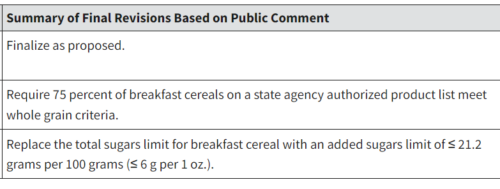
The paper, the press release said, “Illustrate[s] The Need for Nuance in Public Health Guidance Related to Consumption of Sugars: Findings call into question recommendations that imply all sources of fructose-containing sugars carry the same risk.”
The press release notes that “this comprehensive review is timely as the 2025 Dietary Guidelines Advisory Committee currently assesses the latest science to inform updated evidence-based recommendations,” and it quotes the lead author: “There is an opportunity for more food-based guidance around sugars to help ensure Americans don’t inadvertently eat less health-promoting foods containing fructose – especially at a time when most people don’t eat enough of all forms of fruit, which offer significant health benefits.”
Uh oh. This is an easily misinterpreted message.
No surprise.: authors with extensive conflicts of interest.
I’ve written about some of these authors’ conflicts of interest disclosures previously. See, for example. this, this, and this.
Just for fun, I’ll post this particular statement of the conflicted interests at the end of this post.
Basically, these authors do not understand the difference between a conflict of interest (financial ties, which are discretionary) and non-discretionary viewpoints (all researchers have them). In this case, consulting for a sugar company is a conflict; being a vegan or avoiding sugar-sweetened beverages is not.
I contacted John Courtney, the long-time executive director of the ASN. He said this was a leftover from an initiative started ten years ago. Since then, the ASN has decided not to commission papers on controversial topics and this will not happen again.
Good. It shouldn’t. Commissioning papers like these make the ASN look like an arm of the food industry. The ASN should avoid even teh appearance of conflicts of interest as much as it possibly can.
You don’t believe this is a problem? Take a look at this conflict of interest statement. Enjoy!
Conflict of Interest
JLS is a member of the Journal’s Editorial Board and played no role in the Journal’s evaluation of the manuscript.
LC was a Mitacs-Elevate postdoctoral fellow jointly funded by the Government of Canada and the Canadian Sugar Institute (September 2019–August 2021). She was previously (2010–2018) employed as a casual clinical coordinator at INQUIS Clinical Research, Ltd. (formerly Glycemic Index Laboratories, Inc.), a contract research organization.
AC and AA have received funding from a Toronto 3D MSc Scholarship award.
SA-C was funded by a Canadian Institutes of Health Research (CIHR) Canadian Graduate Scholarships Master’s Award, the Loblaw Food as Medicine Graduate Award, the Ontario Graduate Scholarship, and the CIHR Canadian Graduate Scholarship Doctoral Award. She avoids consuming NSBs and SSBs and has received an honorarium from the international food information council (IFIC) for a talk on artificial sweeteners, the gut microbiome, and the risk for diabetes.
NM was a former employee of Loblaw Companies Limited and current employee of Enhanced Medical Nutrition. She has completed consulting work for contract research organizations, restaurants, start-ups, the International Food Information Council, and the American Beverage Association, all of which occurred outside of the submitted work.
TAK has received research support from the Canadian Institutes of Health Research (CIHR), the International Life Science Institute (ILSI), and the National Honey Board. He has taken honorarium for lectures from International Food Information Council (IFIC) and Institute for the Advancement of Food and Nutrition Sciences (IAFNS; formerly ILSI North America).
FA-Y is a part-time Research Assistant at INQUIS Clinical Research, Ltd., a contract research organization.
DL reports receiving a stipend from the University of Toronto Department of Nutritional Sciences Graduate Student Fellowship, University of Toronto Fellowship in Nutritional Sciences, University of Toronto Supervisor’s Research Grant—Early Researcher Awards, and Dairy Farmers of Canada Graduate Student Fellowships; a scholarship from St. Michael’s Hospital Research Training Centre, and a University of Toronto School of Graduate Studies Conference Grant.
AZ is a part-time Research Associate at INQUIS Clinical Research, Ltd., a contract research organization, and has received funding from a BBDC Postdoctoral Fellowship. She has received consulting fees from the GI found.
RJdS has served as an external resource person to the World Health Organization’s Nutrition Guidelines Advisory Group on transfats, saturated fats, and polyunsaturated fats. The WHO paid for his travel and accommodation to attend meetings from 2012–2017 to present and discuss this work. He has also performed contract research for the CIHR’s Institute of Nutrition, Metabolism, and Diabetes, Health Canada, and the World Health Organization for which he received remuneration. He has received speaker’s fees from the University of Toronto and McMaster Children’s Hospital. He has held grants from the Canadian Foundation for Dietetic Research, Population Health Research Institute, and Hamilton Health Sciences Corporation as a principal investigator and is a co-investigator on several funded team grants from the CIHR. He has served as an independent director of the Helderleigh Foundation (Canada). He serves as a member of the Nutrition Science Advisory Committee to Health Canada (Government of Canada) and is a co-opted member of the Scientific Advisory Committee on Nutrition Subgroup on the Framework for the Evaluation of Evidence (Public Health England).
TMSW was previously a part owner and now is an employee of INQUIS and received an honorarium from Springer/Nature for being an Associate Editor of the European Journal of Clinical Nutrition.
CWCK has received grants or research support from the Advanced Food Materials Network, Agriculture and Agri-Foods Canada, Almond Board of California, Barilla, CIHR, Canola Council of Canada, International Nut and Dried Fruit Council, International Tree Nut Council Research and Education Foundation, Loblaw Brands Ltd, the Peanut Institute, Pulse Canada, and Unilever. He has received in-kind research support from the Almond Board of California, Barilla, California Walnut Commission, Kellogg Canada, Loblaw Companies, Nutrartis, Quaker (PepsiCo), the Peanut Institute, Primo, Unico, Unilever, and WhiteWave Foods/Danone. He has received travel support and/or honoraria from the Barilla, California Walnut Commission, Canola Council of Canada, General Mills, International Nut and Dried Fruit Council, International Pasta Organization, Lantmannen, Loblaw Brands, Ltd., the Nutrition Foundation of Italy, Oldways Preservation Trust, Paramount Farms, the Peanut Institute, Pulse Canada, Sun-Maid, Tate & Lyle, Unilever, and White Wave Foods/Danone. He has served on the scientific advisory board for the International Tree Nut Council, the International Pasta Organization, McCormick Science Institute, and Oldways Preservation Trust. He is a founding member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group of the European Association for the Study of Diabetes, is on the Clinical Practice Guidelines Expert Committee for Nutrition Therapy of the EASD and is a Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation.
DJAJ has received research grants from Saskatchewan & Alberta Pulse Growers Associations, the Agricultural Bioproducts Innovation Program through the Pulse Research Network, the Advanced Foods and Material Network, Loblaw Companies, Ltd., Unilever Canada and Netherlands, Barilla, the Almond Board of California, Agriculture and Agri-food Canada, Pulse Canada, Kellogg’s Company, Canada, Quaker Oats, Canada, Procter & Gamble Technical Centre, Ltd., Bayer Consumer Care, Pepsi/Quaker, International Nut & Dried Fruit Council, Soy Foods Association of North America, the Coca-Cola Company (investigator initiated, unrestricted grant), Solae, Haine Celestial, the Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Soy Nutrition Institute (SNI), the Canola and Flax Councils of Canada, the Calorie Control Council, the CIHR, the Canada Foundation for Innovation and the Ontario Research Fund. He has received in-kind supplies for trials as a research support from the Almond Board of California, Walnut Council of California, the Peanut Institute, Barilla, Unilever, Unico, Primo, Loblaw Companies, Quaker (Pepsico), Pristine Gourmet, Bunge Limited, Kellogg Canada, and WhiteWave Foods. He has been on the speaker’s panel, served on the scientific advisory board and/or received travel support and/or honoraria from Nutritional Fundamentals for Health (NFH)-Nutramedica, Saint Barnabas Medical Center, The University of Chicago, 2020 China Glycemic Index International Conference, Atlantic Pain Conference, Academy of Life Long Learning, the Almond Board of California, Canadian Agriculture Policy Institute, Loblaw Companies, Ltd., the Griffin Hospital (for the development of the NuVal scoring system), the Coca-Cola Company, Epicure, Danone, Diet Quality Photo Navigation, Better Therapeutics (FareWell), Verywell, True Health Initiative, Heali AI Corp, Institute of Food Technologists, SNI, Herbalife Nutrition Institute, Saskatchewan & Alberta Pulse Growers Associations, Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Herbalife International, Pacific Health Laboratories, Barilla, Metagenics, Bayer Consumer Care, Unilever Canada and Netherlands, Solae, Kellogg, Quaker Oats, Procter & Gamble, Abbott Laboratories, Dean Foods, the California Strawberry Commission, Haine Celestial, PepsiCo, the Alpro Foundation, Pioneer Hi-Bred International, DuPont Nutrition and Health, Spherix Consulting and WhiteWave Foods, the Advanced Foods and Material Network, the Canola and Flax Councils of Canada, Agri-Culture and Agri-Food Canada, the Canadian Agri-Food Policy Institute, Pulse Canada, the Soy Foods Association of North America, the Nutrition Foundation of Italy, Nutra-Source Diagnostics, the McDougall Program, the Toronto Knowledge Translation Group (St. Michael’s Hospital), the Canadian College of Naturopathic Medicine, The Hospital for Sick Children, the Canadian Nutrition Society, the American Society of Nutrition, Arizona State University, Paolo Sorbini Foundation, and the Institute of Nutrition, Metabolism and Diabetes. He received an honorarium from the United States Department of Agriculture to present the 2013 W.O. Atwater Memorial Lecture. He received the 2013 Award for Excellence in Research from the International Nut and Dried Fruit Council. He received funding and travel support from the Canadian Society of Endocrinology and Metabolism to produce mini cases for the Canadian Diabetes Association. He is a member of the ICQC. His wife, Alexandra L Jenkins, is a director and partner of INQUIS Clinical Research for the Food Industry. His 2 daughters, Wendy Jenkins and Amy Jenkins, have published a vegetarian book that promotes the use of the foods described in this study, The Portfolio Diet for Cardiovascular Risk Reduction (Academic Press/Elsevier 2020 ISBN:978-0-12-810510-8). His sister, Caroline Brydson, received funding through a grant from St. Michael’s Hospital Foundation to develop a cookbook for 1 of his studies. He is also a vegan. JLS has received research support from the Canadian Foundation for Innovation, Ontario Research Fund, Province of Ontario Ministry of Research and Innovation and Science, Canadian Institutes of health Research (CIHR), Diabetes Canada, American Society for Nutrition (ASN), International Nut and Dried Fruit Council (INC) Foundation, National Honey Board [the US Department of Agriculture (USDA) honey “Checkoff” program], Institute for the Advancement of Food and Nutrition Sciences (IAFNS), Pulse Canada, Quaker Oats Center of Excellence, The United Soybean Board (the USDA soy “Checkoff” program), The Tate and Lyle Nutritional Research Fund at the University of Toronto, The Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto (a fund established by the Alberta Pulse Growers), The Plant Protein Fund at the University of Toronto (a fund that has received contributions from IFF), and The Nutrition Trialists Fund at the University of Toronto (a fund established by an inaugural donation from the Calorie Control Council). He has received food donations to support randomized controlled trials from the Almond Board of California, California Walnut Commission, Peanut Institute, Barilla, Unilever/Upfield, Unico/Primo, Loblaw Companies, Quaker, Kellogg Canada, WhiteWave Foods/Danone, Nutrartis, Soylent, and Dairy Farmers of Canada. He has received travel support, speaker fees, and/or honoraria from ASN, Danone, Dairy Farmers of Canada, FoodMinds LLC, Nestlé, Abbott, General Mills, Comité Européen des Fabricants de Sucre, Nutrition Communications, International Food Information Council, Calorie Control Council, the International Sweeteners Association, the International Glutamate Technical Committee, Phynova, and Brightseed. He has or has had ad hoc consulting arrangements with Perkins Coie LLP, Tate & Lyle, Phynova, and INQUIS Clinical Research. He is a former member of the European Fruit Juice Association Scientific Expert Panel and a former member of the Soy Nutrition Institute (SNI) Scientific Advisory Committee. He is on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, European Association for the study of Diabetes, Canadian Cardiovascular Society, and Obesity Canada/Canadian Association of Bariatric Physicians and Surgeons. He serves or has served as an unpaid member of the Board of Trustees and an unpaid scientific advisor for the Carbohydrates Committee of IAFNS. He is a member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group of the EASD, and Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His spouse is an employee of AB InBev.
XYQ, SB, NM, VH, EL, SBM, VLC, and LAL declare no competing interests.
*******
For 30% off, go to www.ucpress.edu/9780520384156. Use code 21W2240 at checkout.