Happy (healthy?) Valentine’s Day!
Happy Valentine’s Day!
It’s a big day for candy sales.

Just for fun, I Googled “healthy Valentine’s Day” just to see what would come up. Lots, it turns out.
Here’s my favorite.

Happy Valentine’s Day!
Happy Valentine’s Day!
It’s a big day for candy sales.

Just for fun, I Googled “healthy Valentine’s Day” just to see what would come up. Lots, it turns out.
Here’s my favorite.

Happy Valentine’s Day!
On Friday December 2 I posted Steve Clapp’s Fixing the Food System in my series on Weekend Reading. His daughter has just written to tell me that Steve died on December 1 of acute leukemia, which he learned he had just six weeks earlier.
I so hope that he was able to see his book, hold it in his hands, and celebrate its arrival.
Memorial service: January 28 at Wakefield Country Day School, Flint Hill, VA, 2:00 p.m. with reception following. Doors open at 1:00.
In sadness, I am repeating the post in honor of his memory.
Steve Clapp. Fixing the Food System: Changing How We Produce and Consume Food. Praeger/ABC-Clio, 2017 (but published November 30).

I wrote the Foreword to this book. Here’s what I said:
In this welcome addition to my library of books about food policy and politics, Steve Clapp’s Fixing the Food System reviews the past and current history of calls for a national food policy, the most contentious controversies over food and nutrition issues that have impeded development of such a policy, and the work of advocates to achieve one. As this book makes clear, this history began decades ago.
I first became aware of the importance of federal food policies in the early 1980s when I was teaching nutrition to medical students at the University of California San Francisco (UCSF). First-year students were eager to learn about nutrition, but for personal more than for professional reasons. They wanted to know what they—and the patients whose health problems they were learning to treat—should eat. But by the time they were residents, I could see their dietary concerns vanish under the daily demands of patient care. Trying to advise about diets was too difficult, time-consuming, and financially unrewarding to be worth the trouble. It seemed unreasonable to expect doctors to take the time needed to counsel individual patients about the prevention of diet-related conditions—heart disease, type 2 diabetes, cancer, and the like. If nutritionists like me wanted to focus on disease prevention rather than treatment, we would have to advocate to change the food environment to make healthful food choices the easy choices—even better, the preferred choices. This meant we would have to advocate for food and nutrition policies aimed at promoting public health.
In 1983, I co-authored an article with UCSF colleagues on the need for such policies.[i] It began:
The U.S. government helps to assure an adequate food supply for Americans by sponsoring a wide variety of food, nutrition, and agricultural support programs. These federal activities were developed in the absence of a clearly articulated national policy, a situation that has resulted in the fragmentation of government programs and their wide disbursement among numerous agencies and departments.
Our article quoted the earliest calls we could find for a national policy to address these problems. In 1974, long before the term “food system” came into common use, the National Nutrition Consortium of four leading nutrition and food science societies[ii] argued for a national nutrition policy that would:
- Assure an adequate, wholesome food supply, at reasonable cost, to meet the needs of all segments of the population.
- Maintain food resources sufficient to meet emergency needs and to fulfill a responsible role as a nation in meeting world food needs.
- Develop a level of sound public knowledge and responsible understanding of nutrition and foods that will promote maximal nutritional health.
- Maintain a system of quality and safety control that justifies public confidence in its food supply.
- Support research and education in foods and nutrition with adequate resources and reasoned priorities to solve important current problems and to permit exploratory basic research.
Whether offered as nutrition or food policies, these were and remain highly appropriate goals for an abundant, healthy, safe, and effective food system.
My co-authors and I went on to identify the constraints that then limited government action to achieve such goals. Despite an emerging consensus on the basic elements of healthful diets—fruits and vegetables, balanced calories, not too much junk food (as Michael Pollan put it more recently, “eat food, not too much, mostly plants”[iii])—the greatest impediment to policy development was the controversy over the science of diet and health. As our article understated this issue,
The effect on the nation’s health of food processing and other changes in the U.S. diet is controversial. Salt, sugar, fiber, saturated fats, alcohol, caffeine, calories, vitamins, and food additives all elicit vigorous debate.
Today, more than 30 years later, we are still arguing about that science, and the scientific arguments still impede policy development. In Fixing the Food System, Steve Clapp brings us up to the minute on federal progress (or the lack thereof) toward achieving a clearly articulated national food policy. He begins and ends his book with the most recent policy proposals from leading food advocates Michael Pollan, of course, but also Mark Bittman, Olivier de Schutter, and Ricardo Salvador. Their recent suggestions for improving our current food system reflect the many changes in agricultural production and food consumption that have taken place since 1974 but retain the basic elements of those earlier proposals. Fixing the Food System explains why a national food policy is so badly needed and matters so much.
Steve Clapp is in a unique position to comment on food policy issues. He’s been at the policy game for a long time. I don’t remember when I first met him but I have been reading his work since he reported for the Community Nutrition Institute’s newsletter, Nutrition Week. For those of us outside the Beltway in those pre-Internet days, Nutrition Week was a lifeline to the ins and outs of food politics in Washington, DC. Later, when Steve moved to Food Chemical News, also—and still—a lifeline, I continued to read his reporting. I often ran across him at meetings and hearings in Washington, DC and found it instructive to read what he wrote about those deliberations, not least because he got it right.
I say all this because he has been a keen observer of the food politics scene in Washington for decades and I can’t think of anyone who ought to know it better. Fixing the Food System reviews the major debates he witnessed—the Dietary Guidelines, of course, but also attempts to set policy for food safety, marketing to children, hunger in America, and humane treatment of farm animals, among others.
Over the years, he also observed the work of policy advocates, and this book includes profiles of many individuals engaged in this work, some likely to be familiar to readers, whereas others may not. Impossible as it is for me to judge whatever impact my own writing and advocacy might have, I am honored to be included among those whose work he presents.
Fixing the Food System describes political arguments over the kind of food system we ought to have and what an ideal system should accomplish. But it is also about the importance of personal and political advocacy for a better food policies, those aimed squarely at promoting public health and environmental sustainability.
Advocacy makes a difference. Advocates are scoring successes in improving one after another aspect of the food system. In comparison to the 1970s or 1980s, we now have better food in supermarkets, more organic foods, more farmers’ markets, more nutritious food in schools, and impressive declines in consumption of sugary drinks. My personal favorite among indicators of advocacy success—the change that makes me most optimistic—is the increasing number of college students who care deeply about food issues. They are demanding local, seasonal, organic, and sustainably produced food in their cafeterias, and campus vegetable gardens. And they are demanding and getting food studies courses and programs like the ones we started at New York University in 1996 that teach about how food is produced and consumed and the practical and symbolic meanings of food in modern culture and societies. Today’s students are tomorrow’s advocates for healthier and more sustainable diets for everyone, everywhere, and for fixing what needs fixing in our food systems. This book is a great starting place for this work.
–Marion Nestle, New York, June 2016
[i] Nestle M, Lee PR, Baron RB. Nutrition policy update. In: Weininger J, Briggs GM, eds. Nutrition Update, Vol. 1. New York: John Wiley and Sons, 1983:285-313.
[ii] National Nutrition Consortium, Inc. Guidelines for a national nutrition policy. Nutrition Reviews 1974;32(5):1253-157. The Consortium included the American Institute of Nutrition, the American Society for Clinical Nutrition, the American Dietetic Association, and the Institute of Food Technology.
[iii] Pollan M. In Defense of Food: An Eater’s Manifesto. Food Rules: An Eater’s Manual. Penguin Press, 2008.
Remember menu labeling—the amazing number of calories posted on your favorite items at chain restaurants? For those of you who don’t live in New York City or other places with calorie labels, they are supposedly coming soon to places near you.
Here’s FDA-speak for what is happening:
In December 2015, section 747 of the 2016 Omnibus Bill prohibited FDA from using appropriated funding to implement, administer, or enforce the menu labeling requirements until one year after FDA finalized the draft September 2015 menu labeling guidance. While FDA originally issued a statement indicating the Omnibus Bill extended the compliance date, FDA is clarifying that the compliance date remains December 1, 2016, but, consistent with the Omnibus Bill, FDA will not begin enforcing the final rule until May 5, 2017, which is one year after the date that the Notice of Availability for the final guidance published in the Federal Register. For more information see: Menu and Vending Machines Labeling Requirements.
Got that?
Really, these are worth waiting for.
Did you really want to eat that 650-calorie muffin?
Thanks today for everything there is to be thankful for, and especially to the National Farmers Union for reminding us how small a share our farmers get of the American food dollar.

I know you can’t read this, so try this piece.
 Or maybe just this one?
Or maybe just this one?

Where does the rest go? Labor, processing, transportation, marketing, etc.
Ponder that, and enjoy your dinner!
Aren’t you happy that it’s that sweet, gooey time of year again?

As Julia Belluz of Vox points out
Candy and Halloween didn’t always go hand in hand. It wasn’t until the 1950s that that candy industry started to push the stuff as a way to boost flagging fall sales.
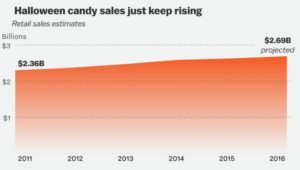
The candy industry would love you to think:
Kids do love candy, as this marketing report tells us. I’ll bet these favorites have everything to do with advertising budgets.

Does candy have a place in healthy diets? Sure, but in very small and occasional amounts.
Good luck getting through tonight’s trick-or-treat.
Happy Halloween, everyone.
Last week, the USDA sent out a press release announcing the last four Final Rules for school meals under the Healthy, Hunger-Free Kids Act (HHFKA) of 2010:
The press release summarizes USDA’s view of what’s most beneficial in these policies:
You probably won’t want to read all the fine print. Fortunately, others have done just that.
Bettina Siegel at The Lunch Tray
Her bottom line:
With the finalization of these four rules, the historic work of the Obama administration in improving children’s school food environment is now complete. But, of course, we’re already one year overdue for the next CNR [Child Nutrition Reauthorization], a process which could easily roll back or weaken these reforms – many of which have already been overtly threatened by House Republicans.
CSPI says the new rules mean local wellness policies can and should:
My comments
Nutritionism: Many of the complaints about USDA’s nutrition standards derive from their focus on single nutrients—fat, salt, sugar—rather than on foods. Boiled eggs weren’t allowed because of their fat and cholesterol content, but copy-cat snack foods were. If the standards applied to minimally processed whole foods, they would make more sense. USDA now has to take comments on whether to eliminate the standard for total fat from Smart Snacks because of the egg issue and the confusing nature of current research on saturated fat (also a problem resulting from studying one nutrient at a time).
Politics: Regardless of how trivial some of these rules may appear, USDA’s school food standards must be considered an extraordinary achievement. Against all odds—unrelenting opposition from companies that supply junk food to schools, Congress, and, weirdly, the School Nutrition Association—the new rules will improve the nutritional quality of school meals and snacks, at least most of the time. School districts with officials who care deeply about improving the food served to kids now have a mandate to do so. Those who don’t will have a harder time doing a bad job. Applause to USDA for bringing the rules to closure. May they survive the next round of lobbying.

And happy thinking. Check out Senator Elizabeth Warren’s speech on industry consolidation and concentration, including what is happening in the food industry.
Competition in America is essential to liberty in America, but the markets that have given us so much will become corrupt and die if we do not keep the spirit of competition strong. America is a country where everyone should have a fighting chance to succeed—and that happens only when we demand it.
Here’s one idea:
The Agriculture Department has a role to play in making sure that poultry farmers and produce growers aren’t held hostage to the whims of giant firms.
The FDA issued its final rules for the Nutrition Facts panels, but now I want to know: What ever happened to its front-of-package (FOP) initiatives?
The New York Times editorial on the new food label raised this very question.
But the labels, which most food companies will have to use by July 2018, still have serious limitations. They require busy shoppers to absorb a lot of facts, not all of which are equally important, and then do the math themselves while standing in the grocery aisle. And the labels are on the back of the package, where only the most motivated shoppers will look.
The editorial refers to the FDA’s front-of-package initiatives early in the Obama administration. These involved two reports from the Institute of Medicine. The first, released in 2010, examined about 20 existing front-of-package schemes cluttering up the labels of processed foods and evaluated their strengths and weaknesses. It recommended that FOP labels deal only with calories, saturated fat, trans fat, and sodium. My question at the time: why not sugars? The committee’s answer: calories took care of it.
But the IOM’s second report in 2011 included sugars and recommended a point system for evaluating the amounts of it and those nutrients in processed foods. Packages would get zero stars if their saturated and trans fat, sodium, or sugars exceeded certain cut points.

The Times editorial explained what happened next:
the Grocery Manufacturers Association [GMA] called the Institute of Medicine’s recommendation “untested” and “interpretive.” Along with the Food Marketing Institute, it developed its own front-of-package labeling system, which includes some useful information, but is more complex and less helpful than the institute’s version.
As I stated at the time, the FDA let the GMA get away with this and has said not one more word about front-of-package labels.
According to the Times, the FDA is still studying the matter.
it’s not clear when or if the agency will require front-of-package labels. The food industry, of course, wants to make its products appear as healthy as possible. The F.D.A. would serve consumers best by taking the Institute of Medicine’s good advice and putting clear and concise nutrition labels right where most shoppers will see them.
It certainly would. It’s time to take those IOM reports out of the drawer and get busy writing rules for them.