Effects of ultraprocessing: fewer phenolics in corn flakes
In FoodNavigator, I read a report of a study finding that processing of corn into breakfast cereal flakes strips out phenolic compounds and tocopherols (vitamin E) associated with good health.
Just as processing of whole wheat into white flour removes the bran and germ, so does the processing of corn into corn flakes.
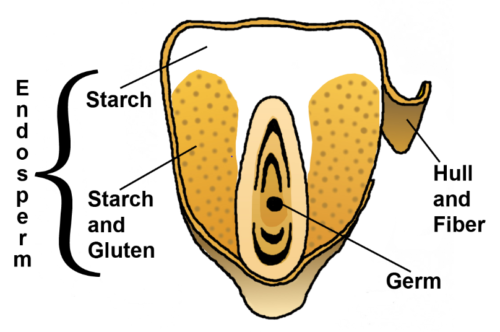
The germ and bran (hull) layers of grain seeds contain the vitamins and minerals—and the phenolics. What’s left is the starch and protein (endosperm).
To replace these losses, manufacturers fortify corn flakes with 10% to 25% of the Daily Value for 12 vitamins and minerals.
This study is further evidence for the benefits of consuming relatively unprocessed foods.
Of particular interest to me is the authors’ disclosure statement:
This work was funded in part through gifts from the Kellogg Company and Dow AgroSciences.
The authors declare no competing financial interest.
This makes this study a highly unusual example of an industry-funded study with a result unfavorable to the sponsor’s interests. The authors do not perceive Kellogg funding as a competing interest. It is. Kellogg (and maybe Dow) had a vested interest in the outcome of this study.
I would love to know whether these authors obtain further research grants from Kellogg and Dow.


