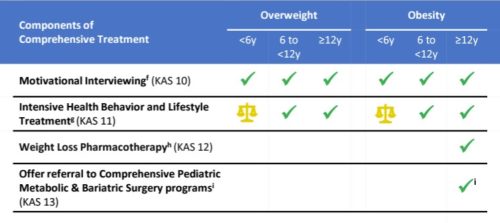
Industry funded study of the week: Beef
Unprocessed red meat in the dietary treatment of obesity: a randomized controlled trial of beef supplementation during weight maintenance after successful weight loss. Faidon Magkos, Sidse I Rasmussen, Mads F Hjorth, Sarah Asping, Maria I Rosenkrans, Anders M Sjödin, Arne V Astrup, Nina R W Geiker. The American Journal of Clinical Nutrition, Volume 116, Issue 6, December 2022, Pages 1820–1830, https://doi.org/10.1093/ajcn/nqac152
Methods: In this 5-mo parallel randomized intervention trial, 108 adults with BMI 28–40 kg/m2 (45 males/63 females) underwent an 8-wk rapid weight loss period, and those who lost ≥8% body weight (n = 80) continued to ad libitum weight maintenance diets for 12 wk: a moderate-protein diet with 25 g beef/d (B25, n = 45) or a high-protein diet with 150 g beef/d (B150, n = 35).
Conclusions: Healthy diets consumed ad libitum that contain a little or a lot of unprocessed beef have similar effects on body weight, energy metabolism, and cardiovascular risk factors during the first 3 mo after clinically significant rapid weight loss.
Funding: The study was supported by The Beef Checkoff (a program of the National Cattlemen’s Beef Association, CO, USA) and the Danish Agriculture & Food Council (Copenhagen, Denmark). Lighter Life (Essex, UK) sponsored very-low-calorie diet products for the weight-loss phase of the study. The sponsors had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Conflicts of interest: NRWG has received funding from The Beef Checkoff program (National Cattlemen’s Beef Association, CO, USA) and the Danish Agriculture & Food Council (Copenhagen, Denmark) to conduct additional studies relevant to the role of meat in the diet. AVA is a member of the scientific advisory board for Weight Watchers, USA; congressional chairman for RNCP (Répertoire National des Certifications Professionnelles), France; co-owner of the University of Copenhagen spin-off Flax-Slim ApS; co-inventor on a pending provisional patent application for the use of biomarkers to predict responses to weight-loss diets; and co-inventor of other related patents and patent applications that are all owned by the University of Copenhagen, in accordance with Danish law. All other authors report no conflicts of interest.
Comment: The conclusion of this beef industry-funded study is that you can eat as much beef as you like without its having any effect on your body weight or metabolic risk factors, as long as you first lose weight and keep it off. This is a perfect example of why looking at one food at a time makes no sense without also taking into consideration everything else you are eating and how much. The Beef Checkoff got the answer it wanted, so money well spent.